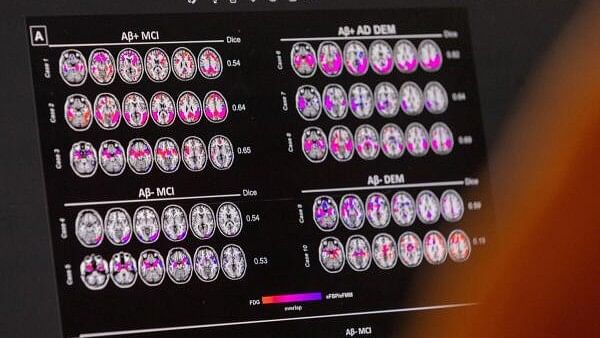
Hypometabolic and hypoperfusion patterns at the single-subject level from a patient suffering from Alzheimer's.
Credit: Reuters
By Lisa Jarvis
In a new paper in Nature Medicine, an international team of neurologists makes the compelling case that people with two copies of a gene called APOE4 aren’t just at risk of Alzheimer’s — they have a distinct form of the disease and are almost certain to develop its telltale brain plaques by age 65.
The finding comes with caveats, but still has near-term implications for studying, diagnosing and treating the disease — especially given the advent of drugs like Leqembi, made by Eisai and Biogen, and donanemab, made by Eli Lilly & Co. It should also motivate the field to push further into treatments that specifically target the protein encoded by this gene.
It also raises a critical question for the public: Should more of us know whether we are carriers of these genes?
Researchers have known for decades that people who harbor APOE4 have a higher risk of developing the neurological condition. One copy raises the chances of getting the disease as much as threefold, while two copies increases the risk by a factor of 10 or more. Many will recall movie star Chris Hemsworth’s 2022 revelation that he carried two copies of the gene, news that reportedly prompted him to approach his life with a bit more intention.
This new study puts a finer point on that risk. Using two datasets that encompass over 10,000 patients and over 3,000 brain donors, the researchers show that people with two copies of the gene will almost inevitably develop biological signs of the disease. Some 95 per cent had build-up of a protein called amyloid, a hallmark of Alzheimer’s disease, in their brains and spinal fluid by the time they are 65. For that reason, the authors argue, it should be considered a distinct genetic form of the disease.
Graph showing increase of Alzheimer's patients in US.
Credit: Bloomberg
Those data make it tempting to consider these gene variants as causing the disease. But it’s worth stopping short of that — for now, at least. One big stumbling block is that while the study makes clear that nearly everyone in this group has amyloid plaque on their brains, we still can’t predict when they’ll develop symptoms like confusion and forgetfulness, if at all.
The other big caveat is that the people included in the study all came from the US or Europe, and were predominantly White, leaving open the question of whether having two copies of the gene is a cause of the disease or if other genetic and environmental factors contribute to its development in this population, points out Jason Karlawish, co-director of the Penn Memory Center at the University of Pennsylvania.
Still, Karlawish said via email, it’s “a provocative study that posits a conclusion, which, if verified, will transform how we categorize, diagnose and treat Alzheimer’s disease.”
The most immediate impact could be on rethinking when and how to use approved and experimental drugs. This concrete evidence that Alzheimer’s can be detected in the brain years before symptoms start “is an incredible opportunity to try to intervene,” says Reisa Sperling, director of the Center for Alzheimer Research and Treatment at Brigham and Women’s Hospital, and an author on the study. The benefits could be significant: Some 2–3 per cent of the population has two copies of APOE4, and it accounts for as many as 15–20 per cent of the people with Alzheimer’s.
But intervening with existing drugs will be a bit tricky. Although the Food and Drug Administration last year approved Leqembi, a drug that clears amyloid plaques, it carries a warning that it can cause serious side effects in people with two copies of APOE4. And Lilly’s donanemab, which is currently under FDA review, carries a similar risk.
Patients with two copies of the gene are more likely to experience dangerous brain swelling or bleeds if taking the drugs. And in clinical studies, Leqembi didn’t do much to slow their cognitive decline, likely because so much amyloid had built up on their brains by the time they’d been treated. Sperling thinks these new data suggest it’s worth using these drugs in this group of patients — but earlier, very carefully, and ideally within the context of clinical trials focused on prevention.
Future studies should separate out people with one and two copies of APOE4, as their disease trajectory differs. Careful study of these patients over time could also offer broad lessons about how the disease manifests — and where and when to intervene. And more effort should be put into treatments tailored to people harboring these genes. A small pipeline has emerged of APOE4-targeted treatments, including gene therapies designed to increase production of APOE2, a form of the protein that is known to be protective against the disease.
The public will undoubtedly wonder whether this means they should go out and get tested for the gene.
The question is one of deep debate. On the one hand, without a treatment to disrupt the course of the disease, the results might simply cause anxiety. On the other, people could make lifestyle changes, prepare for a future where they might need long-term care, or even seek out a clinical trial.
It would be worth being more liberal in testing for people showing up at their doctor’s offices with vague symptoms, even if they’re young. And certainly, testing should become widespread if studies can untangle when and why the biological signs will lead to cognitive decline.
In the meantime, it’s worth being hopeful: Drug development is advancing and studies like these could speed not only treatments for Alzheimer’s, but ways to prevent it.