Over the last few days, India has been reporting more than one lakh Covid-19 cases daily. The country has been witnessing a record high number of cases, more than last year when the pandemic first began.
While the government has been imposing curbs in various states, the only other way to beat the virus would be to ramp up the vaccination drive in the country. While India has a mass vaccination drive on, there seems to be a vaccine shortage in some states.
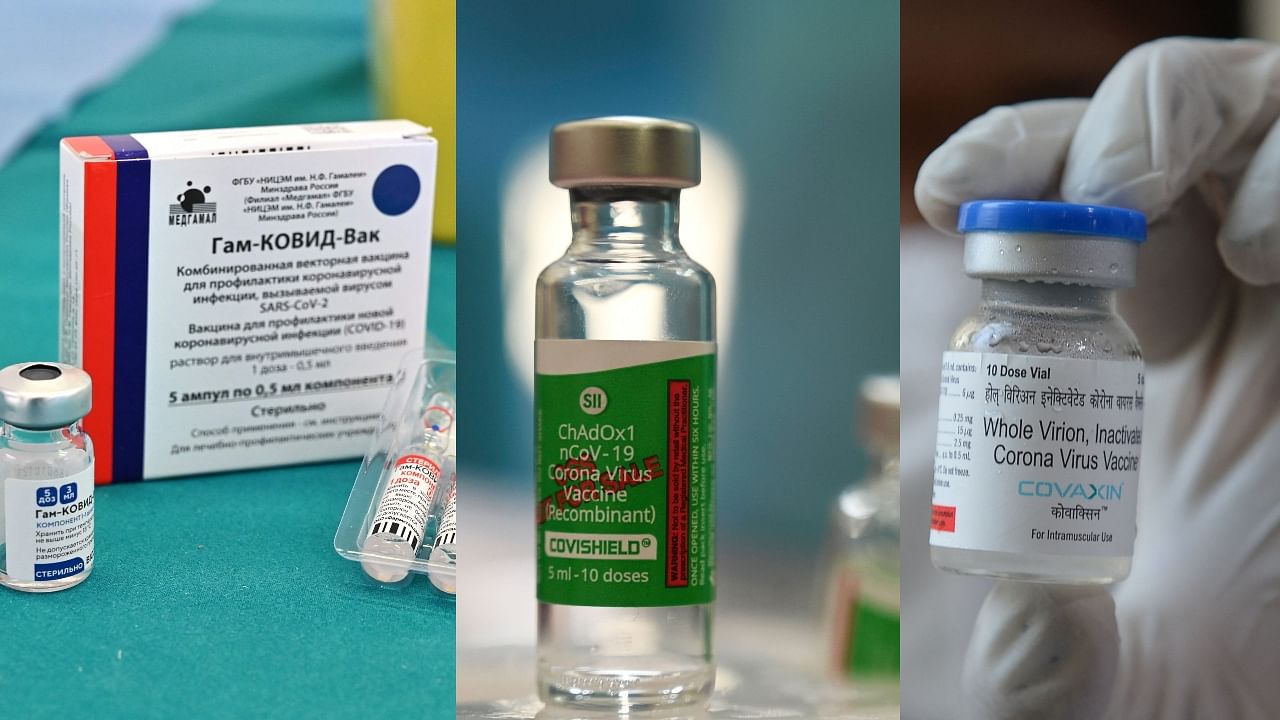
To this effect, the Drug Controllers General of India approved a third Covid-19 vaccine in the country - Russia's Sputnik V. Prior to this, India had only two vaccines, Bharat Biotech's Covaxin, and Oxford-AstraZeneca's Covishield, the latter is being manufactured locally by the Serum Institute of India.
Let's take a look at the differences in the three Covid-19 vaccines in the country.
Sputnik V
Russia's Sputnik V vaccine, named after the Moscow's first artificial satellite, was developed by Moscow's Gamaleya Institute. This vaccine uses two different viruses, which are responsible for common cold in humans. The viruses are then modified in a way that makes coronavirus spike protein. This way it safely exposes the body to a part of the Covid genetic code, allowing the body to recognise it and fight it off. After being vaccinated, the body starts to produce antibodies, especially, tailored for the coronavirus.
The dosage of Sputnik V includes two dates administered 21 days apart. Unlike other vaccines, the two doses of Sputnik have different versions. They both target the coronavirus' distinctive "spike", but use different vectors - the neutralised virus that carries the spike to the body. This is done because different formulas would prevent the body from developing an immune response to the first shot and reacting negatively to the booster shot.
The Russian Direct Investment Fund (RDIF), which is marketing the vaccine, said that India will produce 850 million Sputnik V doses annually
The Sputnik V is easy to store and transport since it can be stored at temperatures of between 2 and 8 degrees Celsius.
The efficacy rate of Sputnik V is around 92 per cent against Covid-19, as per late stage trial results published in the Lancet.
The side effects of the vaccine have been mild, and include a sore arm, tiredness and a bit of a temperature. There were no deaths or serious illnesses in the vaccinated group linked to the jab
India is now the 60th country to have approved Russia's Sputnik V following countries like Argentina, Palestinian territories, Venezuela, Hungary, UAE and Iran.
Covaxin:
Covaxin is made of killed coronaviruses, or inactivated virus which is to be injected into the body. Immune system recognises dead virus and makes antibodies against it. The vaccine is to be administered in two doses, given four weeks apart.
Bharat Biotech, the manufacturer, says it has a stockpile of 20 million doses of Covaxin, and is aiming to make 700 million doses out of its four facilities in two cities by the end of the year.
The vaccine can be stored at 2- 8 degrees Celsius, and has an efficacy rate of 81 per cent, as per preliminary data from its phase 3 trials.
Side effects that have been reported with Covaxin include, but are not limited to, injection site pain, swelling, redness, itching, headache, fever, malaise/body ache, nausea, vomiting rashes. Bharat Biotech also warns that serious and unexpected side effects may occur.
Covishield:
The Oxford-AstraZeneca vaccine, being manufactured locally by the Serum Institute of India, is producing more than 60 million doses a month. Covishield is made from a weakened version of the common cold (known as adenovirus) following the same pattern of production like Sputnik V. The weakened version has been modified to look more like coronavirus - although it cannot cause illness.
As is the case with all vaccines, once injected, it prompts the immune sytem to make antibodies, and prevent any coronavirus infection.
The Covishield jab is administered in two doses between four and 12 weeks apart, and can be safely stored at temperatures of 2-8 degrees Celsius.
According to international clinical trials of the Oxford-AstraZeneca vaccine, when people were given a half dose and then a full dose, effectiveness hit 90 per cent. But there is not enough data to support the statement.
However, unpublished data suggests that leaving a longer gap between the first and second doses increases the overall effectiveness of the jab. The vaccine this way was found to be 70 per cent effective after the first dose.
Side effects that have been reported with the Covishield vaccine include, but are not limited to, tenderness, pain, warmth, redness, itching, swelling, or bruising where the injection is given; generally feeling unwell, feeling tired (fatigue), chills or feeling feverish, headache, feeling sick (nausea), joint pain or muscle ache. Less common side effects were reported to be a lump at the injection site, fever, being sick (vomiting), flu-like symptoms, such as high temperature, sore throat, runny nose, cough and chills. And some uncommon symptoms were feeling dizzy, decreased appetite, abdominal pain, enlarged lymph nodes, and excessive sweating, itchy skin or rashes.
India has shipped close to 64 million doses of vaccines to 86 countries in Latin America, the Caribbean, Asia and Africa.
Both Covishield and Covaxin have been exported either in the form of "gifts", or under guidelines and regulations of the World Health Organisation (WHO).
But earlier this month, India placed a temporary hold on all exports of the Covishield. The government said rising cases meant domestic demand was expected to pick up and so the doses were needed for India's own rollout.