India vows to decide on foreign vaccines within 3 days to fast-track importsCurrently, two vaccines -- Covaxin by Bharat Biotech and Covishield by Serum Institute of India (SII) -- are being used for inoculation in India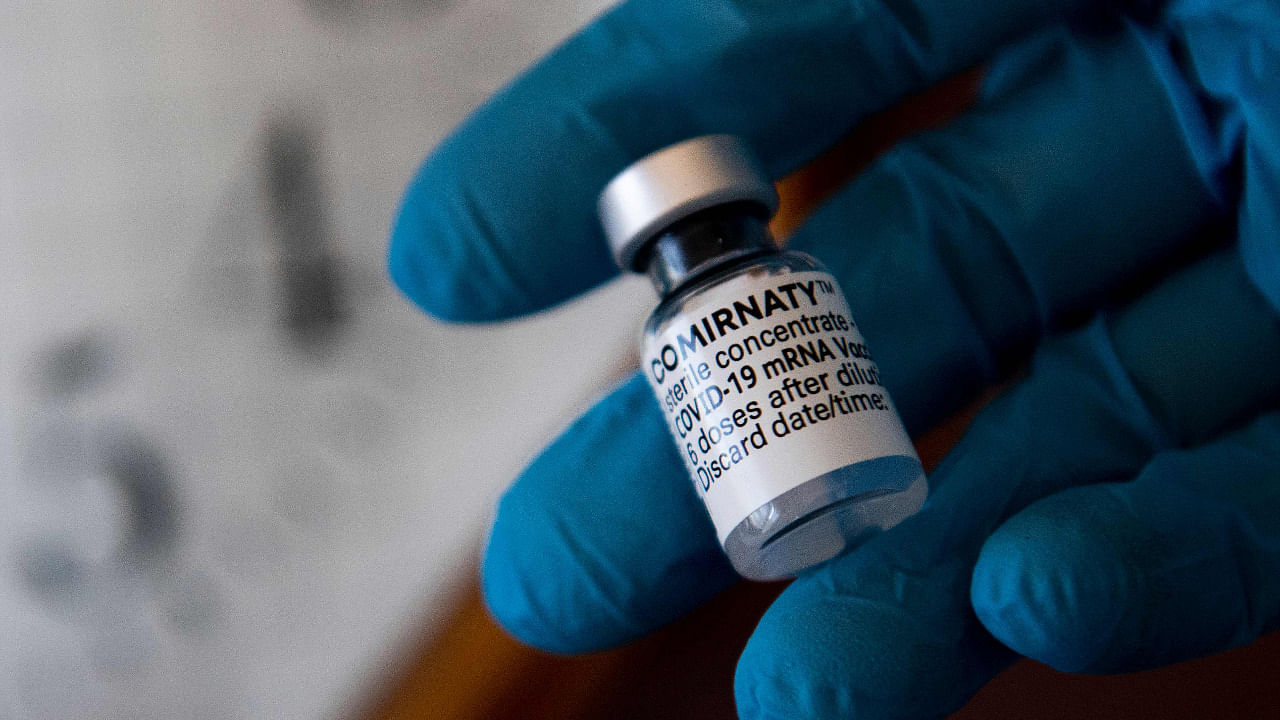
Last Updated IST
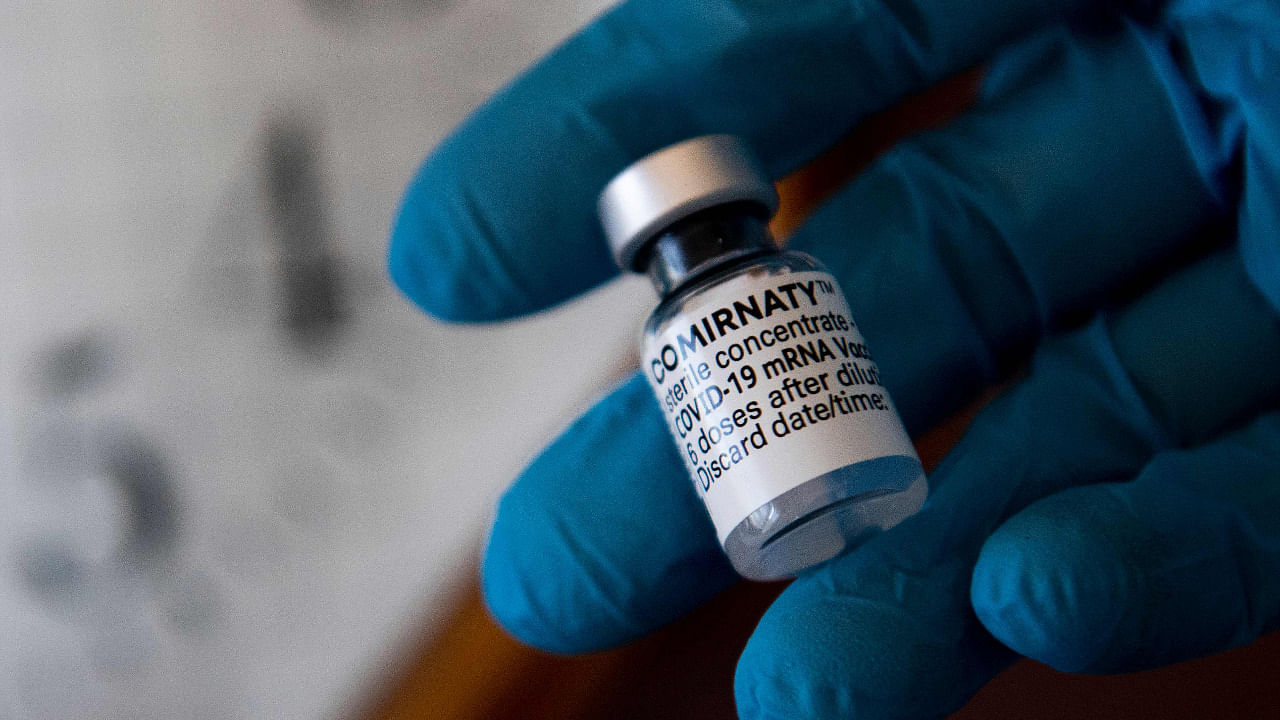
A dose of a single-shot Comirnaty Pfizer-BioNTech Covid-19 vaccine. Credit: AFP Photo
India said on Friday its drugs regulator will decide on emergency-use applications for foreign Covid-19 vaccines within three working days from application, as it tries to attract Pfizer, Johnson & Johnson and Moderna to sell their shots.
Also Read | Govt fast-tracks approval for foreign-produced Covid-19 vaccines cleared in other countries
The regulator "will process such applications for Restricted Use in Emergency Situation" and its chief "will consider and take a decision within 03 working days from date of submission of complete application by the applicant," the health ministry said in a statement.