Maharashtra drug regulator orders sample checks on all oral liquid solutions: ReportThe move comes after production was halted at New Delhi-based Maiden Pharmaceuticals following cough syrup-related deaths in The Gambia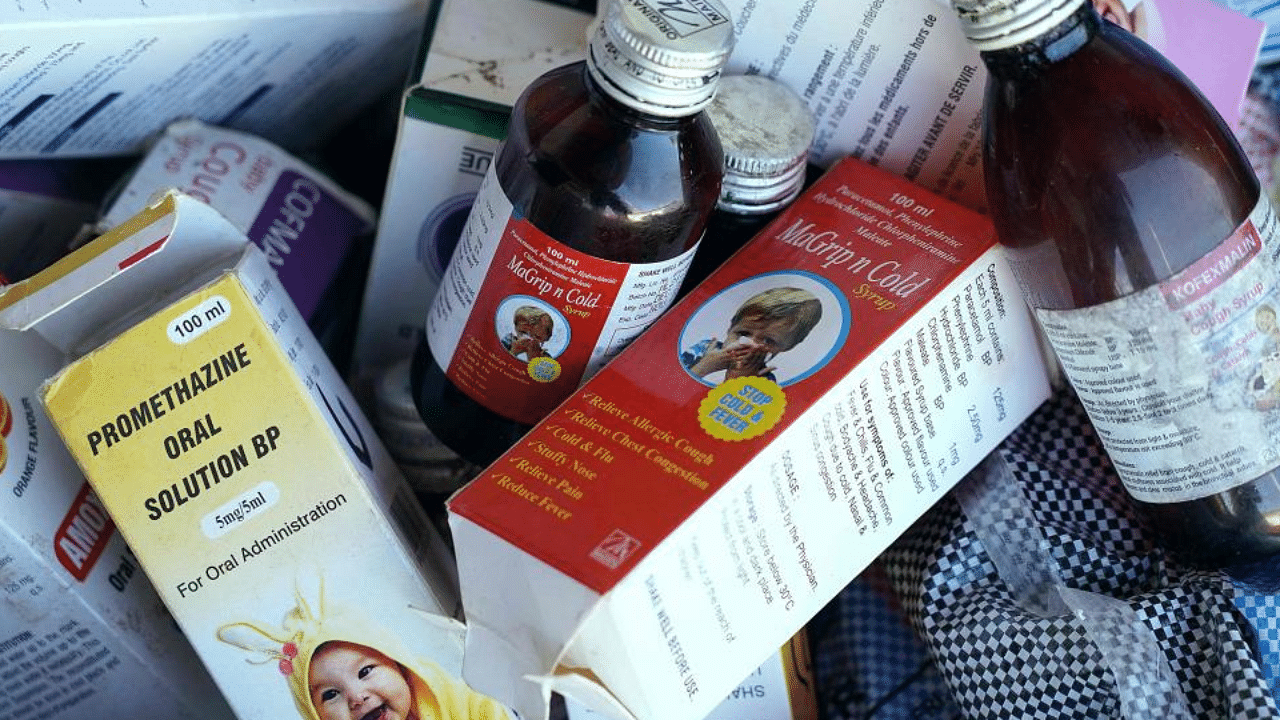
Last Updated IST

A photograph shows collected cough syrups in Banjul. Credit: AFP Photo
The drug regulator of Maharashtra has ordered sample checks on all oral liquid solutions in the state, asking for reports on the levels of diethylene glycol and ethylene glycol, CNBC TV18 reported on Thursday.
The regulator's direction comes a day after Indian health authorities said they had halted all production of New Delhi-based Maiden Pharmaceuticals following a WHO report that its cough and cold syrups exported to Gambia may be linked to the deaths of dozens of children there.