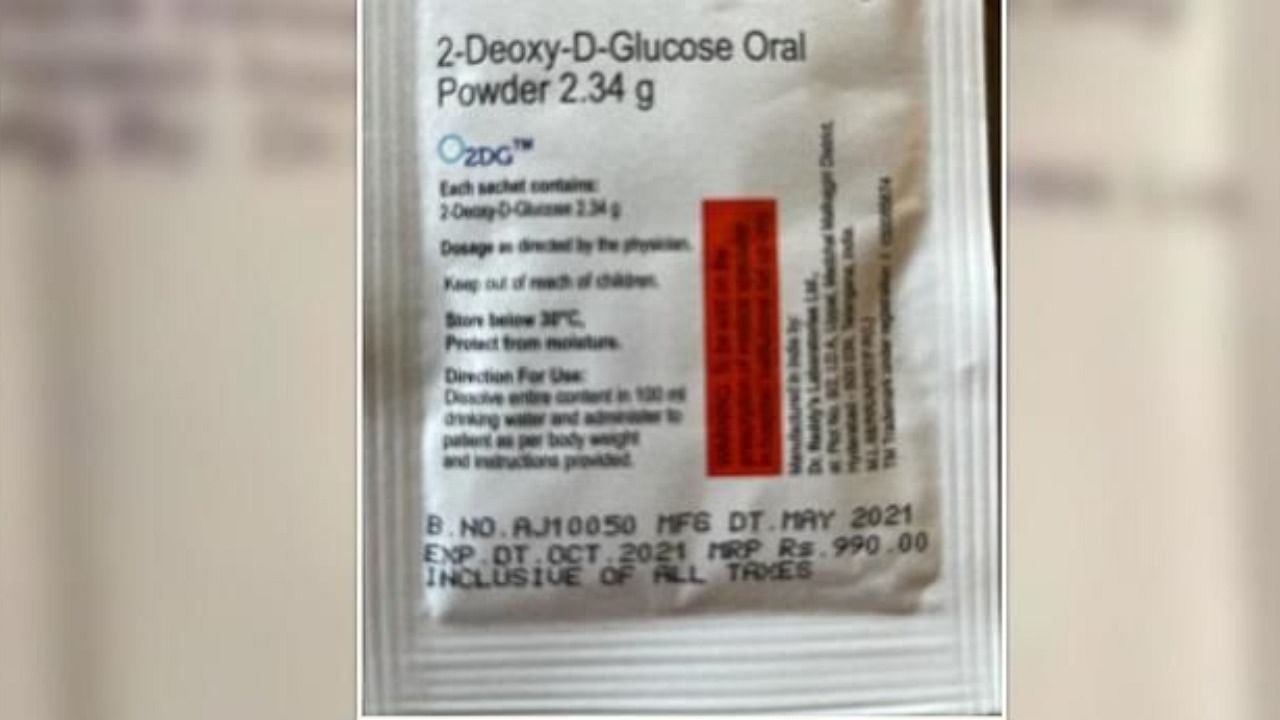
Drug firm MSN Laboratories on Friday said it has entered into a licence agreement with the Defence Research & Development Establishment (DRDE) for the manufacturing, distribution and marketing of 2-Deoxy-D-Glucose (2-DG), used for the treatment of Covid-19.
Developed by DRDO, 2-DG has been granted permission by the Drug Controller General of India (DCGI) for emergency use as adjunct therapy in moderate to severe Covid-19 patients, MSN Labs said in a statement.
The company has entered into a licence agreement with DRDE and the Institute of Nuclear Medicine and Allied Sciences (INMAS) establishments of DRDO for the manufacturing, distribution and marketing of 2-Deoxy-D-Glucose (2-DG) in India, it added.
MSN labs will be launching the 2-DG as a twice-a-day product in sachet form under the brand name MSN 2D in strength of 2.34 g, the statement said.