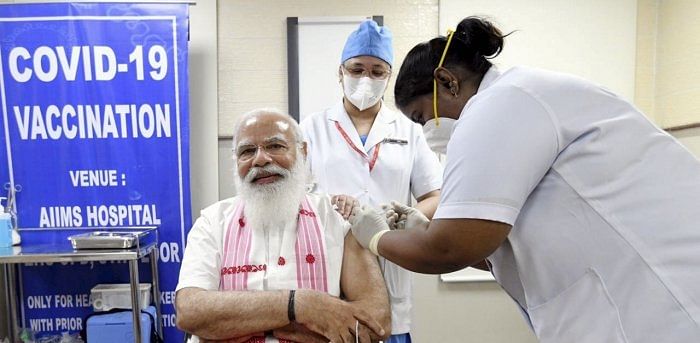
Bharat Biotech International Limited (BBIL), the maker of Covaxin which was administered to Prime Minister Narendra Modi on Monday said that the gesture has “set a powerful example for all Indians to follow.”
The nationwide Covid-19 vaccination programme, set in motion on 16 January, is from Monday onwards catering to the common public, and will first cover senior citizens and those of 45 years and above with co-morbid conditions.
The Hyderabad based biotech firm has said that the PM's move “will go a long way in reducing vaccine hesitancy.”
Last year, BBIL developed BBV152 or Covaxin in collaboration with the Indian Council of Medical Research-National Institute of Virology. The indigenously developed inactivated, two-dose SARS-CoV2 vaccine is included in the ongoing Covid-19 vaccination programme in the country.
Read: Politicians known to be thick-skinned, says PM Modi
Covaxin was given the emergency use authorization by the central drug authorities in early January even as it was in Phase-3 trials, and amid uncertainty over its efficacy.
Covishield, developed by Oxford University- AstraZeneca, and manufactured by the Serum Institute of India, Pune is also part of the ongoing vaccination drive.
However, PM Modi chose to take the first dose of the homegrown vaccine on Monday at AIIMS, New Delhi, to mark the beginning of the Phase-2 Covid-19 immunization programme.
Dr Krishna Ella, Chairman & Managing Director, Bharat Biotech said, “We express our sincere gratitude to the Prime Minister for reposing his trust in the indigenously developed Covid-19 vaccine, COVAXIN. We urge all fellow citizens to not hesitate from taking part in the immunization programme so that the country can bring an end to this public health crisis.”
Read more: What PM told the nurse who gave him Covid-19 vaccine
“We thank Prime Minister Modi for taking the first dose of Covid-19 vaccine himself. We are deeply appreciative of the gesture which has set a powerful example for all Indians to follow. This would go a long way in reducing vaccine hesitancy and building confidence in immunization against the ongoing pandemic,” Ella further said.
BBIL said that it is partnering with various countries on Covaxin supply, including a deal reached last week to ship 20 million doses to Brazil.
BBV152 (COVAXIN) contains a whole virion inactivated SARS-CoV-2 vaccine, which is produced in Vero cells. The vaccine is stable at +2°C to +8°C (refrigerated) and is shipped in a ready-to-use liquid formulation that permits distribution using existing vaccine supply chain channels, the company said.